39 labelled diagram of ph scale
Acids, Bases, & the pH Scale - Science Buddies In order to deal with these large numbers more easily, scientists use a logarithmic scale, the pH scale. Each one-unit change in the pH scale corresponds to a ten-fold change in hydrogen ion concentration. The pH scale is theoretically open-ended but most pH values are in the range from 0 to 14. pH Scale - PhET pH Scale - PhET
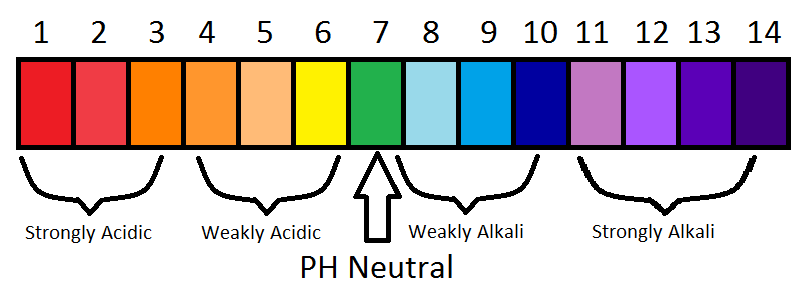
Draw a neat and labelled diagram of pH scale class 10 chemistry CBSE Hint : We all are familiar with the term p H and p H scale. p H scale is a scale of acidity and basicity which tells about the acidic and alkaline behaviour of substance . > On the p H scale the value ranges from 0 to 14. So the more acidic solution will have lower p H and neutral solutions will have p H equal to 7 and more than 7 acidic nature ...

Labelled diagram of ph scale
Label The Ph Scale Diagram / Ph Scale Infographic Acidbase Balance ... Start studying label the ph scale. Ph less than 7 corresponds to acidic . As this diagram shows, ph ranges from 0 to 14, with 7 being neutral. A ph of 7 is neutral. Phs less than 7 are acidic while phs greater than 7 are alkaline . Jazzirt/getty images the usual range of ph values runs from 0 to 14. The PH Scale Universal Indicator PH Color Chart Diagram Acidic ... - 123RF Illustration of The pH scale Universal Indicator pH Color Chart diagram acidic alkaline values common substances vector illustration flat icon design Colorful vector art, clipart and stock vectors. Image 81803308. pH Scale With Examples - Labelled diagram - Wordwall pH Scale With Examples - Labelled diagram Neutral, Weak Acid, Strong Acid, Weak Alkali, Strong Alkali, Battery Acid, Stomach Acid, Lemon Juice, Carbonated Drinks, Tomato Juice, Coffee, Milk, Water.
Labelled diagram of ph scale. PH Meter Definition, Principle, Parts, Types, Application, Procedure. Portable pH meter: covering an extensive range of regularly employed instruments, the exception is the use of compact DC power equipment can be produced to the scene. Desktop pH meter: Same as Portable pH meter. Pen pH meter: normally composed of a single scale, conventional measurement range, for the easy and handy equipment. The pH scale - BBC Bitesize The pH scale is a number scale from 0 to 14. It tells us how acidic or alkaline an aqueous solution is. The pH scale is used to classify as acidic, alkaline or neutral. Neutral solutions are... pH Calculator | How To Calculate pH? The pH scale (pH) is a numeric scale which is used to define how acidic or basic an aqueous solution is. It commonly ranges between 0 and 14, but can go beyond these values if sufficiently acidic/basic. pH is logarithmically and inversely related to the concentration of hydrogen ions in a solution. The pH to H + formula that represents this relation is: ... Acids, Alkalis, and the pH Scale - Compound Interest A pH of spot on 7 denotes a neutral solution (neither acidic or alkaline). Any pH below 7 is acidic, whilst any pH above 7 is termed alkaline. Water molecules have the chemical formula H 2 O. However, these molecules are capable of splitting up slightly in solution, in H + and OH - (hydroxide) ions.
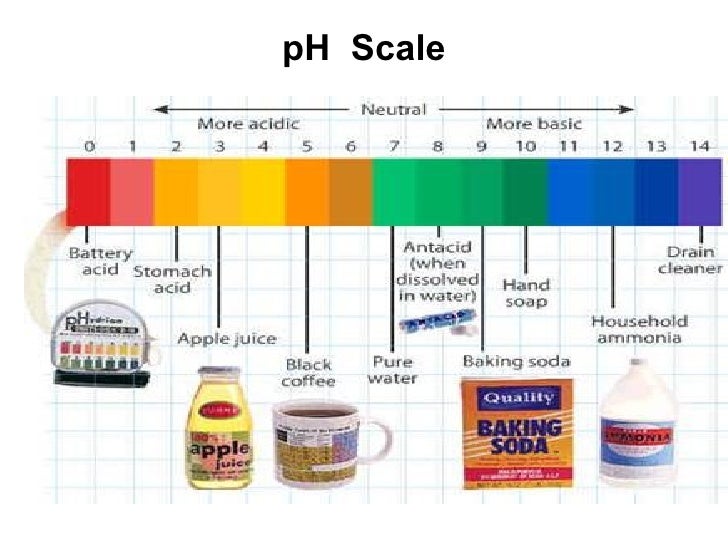
pH Scale - Elmhurst University pH Scale: The pH scale, (0 - 14), is the full set of pH numbers which indicate the concentration of H + and OH - ions in water. The diagram on the left gives some relationships which summarizes much of the previous discussion. pH Scale Principle: H+ ion concentration and pH relate inversely. OH- ion concentration and pH relate directly. pH (TITRATION) CURVES - chemguide Simple pH curves. All the following titration curves are based on both acid and alkali having a concentration of 1 mol dm-3.In each case, you start with 25 cm 3 of one of the solutions in the flask, and the other one in a burette.. Although you normally run the acid from a burette into the alkali in a flask, you may need to know about the titration curve for adding it the other way around as well. Diagram of the pH scale with examples of acidic, neutral and alkaline ... File size: 36.7 MB (381.7 KB Compressed download) Releases: Model - no | Property - no Do I need a release? Dimensions: 4708 x 2724 px | 39.9 x 23.1 cm | 15.7 x 9.1 inches | 300dpi Photographer: Spencer Sutton More information: Diagram of the pH scale with examples of acidic, neutral and alkaline substances. Search stock photos by tags Show all Draw Neat and Labelled Diagram of pH Scale. - Shaalaa.com Draw Neat and Labelled Diagram of pH Scale. Maharashtra State Board SSC (Marathi Semi-English) 10th Standard [इयत्ता १० वी] Question Papers 172. Textbook Solutions 10056. MCQ Online Tests 30. Important Solutions 1991. Question Bank Solutions 7377. Concept Notes & Videos & Videos 327.
The pH scale with some common examples The pH scale, with examples of common solutions and their pH values. Download/View For commercial use please contact us Draw neat and labeled diagram of pH scale? - Toppr Ask The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 pH meter - Wikipedia A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH electrode and a reference electrode, and so the pH meter is sometimes referred to as a "potentiometric pH meter". The difference in electrical potential relates to ... pH Scale Flashcards | Quizlet pH. abbreviation meaning "potential for hydrogen." pH Scale. a scale used to define the levels of hydrogen (H) or hydroxide (OH) ions in a solution. Ranges from 0 (acidic) to 14 (basic/alkaline), with 7 being neutral. Hydrogen Ion. an ion of hydrogen (H) created by the breaking up of a water molecule during the mixing of a solution.
What is pH? - BYJUS At 100°C, a pH value of 6.14 is the new neutral point on the pH scale at this higher temperature. pH of Acids and Bases. The pH of a solution varies from 0 to 14. Solutions having a value of pH ranging 0 to 7 on pH scale are termed as acidic and for the value of pH ranging 7 to 14 on pH scale are known as basic solutions.
Label The Ph Scale Diagram / Science Worksheets Label Color The Ph ... The neat and labeled diagram of ph scale is as shown. a diagram a b diagram b c diagram c d diagram d. Ph 7 corresponds to neutral ph. As this diagram shows, ph ranges from 0 to 14, with 7 being neutral. Students then color the given ph scale reflected in ph indicator pap. Find out what negative ph means. If you are given the molarity of hydrogen.
pH Scale | U.S. Geological Survey - USGS.gov Each number represents a 10-fold change in the acidity/basicness of the water. Water with a pH of five is ten times more acidic than water having a pH of six. As this diagram shows, pH ranges from 0 to 14, with 7 being neutral. pHs less than 7 are acidic while pHs greater than 7 are alkaline (basic). Learn more about pH Sources/Usage
Chemistry: pH scale Diagram | Quizlet Basic (Alkaline) A solution with a pH above 7. Greater concentration of hydroxide ions than concentration of hydrogen ions. pH scale according to the universal pH indicator strip Red is acidic; green is neutral; blue is basic/alkaline 1M hydrocloric acid (HCl) pH0 1M Sodium Hydroxide (NaH) pH14 Water (H2O) pH7 THIS SET IS OFTEN IN FOLDERS WITH...
Solved Drag the labels onto the diagram to identify the - Chegg Transcribed image text: Drag the labels onto the diagram to identify the various components of the pH scale. Reset Increasing concentration of OH Extremely basic Pure water Neutral Increasing concentration Stomach acid Blood Household ammonia Extremely acidic of Urine 1 mol/L hydrochloric acid Beer vinegar wine, Tomatoes, pickles grapes 1 moll sodium hydroxide Oven deaner Saliva milk Ocean ...
Acids & Bases - pH Scale - Labelled diagram - Wordwall Strong Acid, Moderate Acid, Weak Acid, Neutral, Weak Base, Moderate Base, Strong Base, pH 0-2, pH 3-4, pH 5-6, pH 7, pH 8-9, pH 10-11, pH 12-14, Hydrochloric Acid (HCl), Vinegar (Acetic acid), Lemon juice (Citric Acid), Water, Sodium bicarbonate (baking soda), Ammonia, Sodium hydroxide (NaOH). Acids & Bases - pH Scale Share Share
Label The Ph Scale Below In Terms Of Acid Base And Neutral - Back To ... The range of ph is from 0 to 14. As this diagram shows, ph ranges from 0 to 14, with 7 being neutral. Label each solution as acidic, basic, or neutral based only on the stated ph. The ph scale measures how . Acids have ph values below 7;; The neat and labeled diagram of ph scale is as shown. Distilled water is a neutral substance.
The pH Scale - Introductory Chemistry - 1st Canadian Edition Label each solution as acidic, basic, or neutral based only on the stated pH. milk of magnesia, pH = 10.5 pure water, pH = 7 wine, pH = 3.0 Solution With a pH greater than 7, milk of magnesia is basic. (Milk of magnesia is largely Mg (OH) 2 .) Pure water, with a pH of 7, is neutral. With a pH of less than 7, wine is acidic. Test Yourself
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. pH scale, ranging from 0 (very acidic) to 14 (very basic/alkaline) and listing the pH values of common substances.





Post a Comment for "39 labelled diagram of ph scale"