43 label the molecules as cis or trans.
Solved Consider the molecule. Label molecule A as cis or | Chegg.com Label molecule A as cis or trans. Br trans O cis does not apply CI Molecule A Consider the molecule. Label molecule B as cis or trans. + O trans does not apply O cis CI Br Molecule B Consider the molecule. Label molecule C as cis or trans. Br O does not apply O trans O cis CI Molecule C Consider the molecule. › templates › categoryAll Label Templates | Avery.com All Label Templates; All Label Templates. Avery Templates by Product Number. Search by product number. See all. 0 items. FILTER . SORT BY: Most Popular . Most Popular Product #: Low - High Product #: High - Low Size: Small - Large Size: Large - Small . For the latest trends, ideas & promotions.
learn.microsoft.com › en-us › windows-serverlabel | Microsoft Learn Mar 3, 2021 · An NTFS volume label can be up to 32 characters in length, including spaces. NTFS volume labels retain and display the case that was used when the label was created. Examples. To label a disk in drive A that contains sales information for July, type: label a:sales-july To view and delete the current label for drive C, follow these steps:

Label the molecules as cis or trans.
Answered: Label the molecules as cis or trans. Br… | bartleby Label the molecules as cis or trans. Br B) CI 2 Br 6 Br D) -0 Br Question Transcribed Image Text: Label the molecules as cis or trans. Br B) CI does not apply trans cis Br trans cis does not apply 6 Br cis does not apply trans D) -G Br does not apply trans cis Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution Solved Label the molecules as cis or trans. Trans cis - Chegg Question: Label the molecules as cis or trans. Trans cis does not apply trans does not apply cis trans cis does not apply trans does not apply cis This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Show transcribed image text Expert Answer 100% (5 ratings) Label the molecules as cis or trans. Trans cis does not apply trans ... Answer 2:- C) Cis-2-pentene, because its molecules cannot stack as efficiently Explanation:- in melting point... Answer true or false. (a) Both ethylene and acetylene are planar molecules. (b) An alkene in which each carbon of the double bond has two different groups bonded to it will show cis-trans isomerism.
Label the molecules as cis or trans.. Cis-Trans Isomers - Definition, Detailed Explanation with Examples - BYJUS The cis and trans isomers of butenedioic acid display very different reactivities, which can be attributed to the difference in their properties. Maleic acid is the cis isomer and fumaric acid is the trans isomer. Elaidic acid and oleic acid are cis-trans isomers. Answered: Label the following molecules as in the… | bartleby Label the following molecules as in the cis or trans configuration? Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution star_border Students who've seen this question also like: Chemistry Chemical Foundations. 1RQ expand_more Want to see this answer and more? . Label each of the following as cis or trans If no cis/trans... Label each of the following as cis or trans If no cis/trans isomerism is possible for a given compound, indicate "not possible". ... Isomers are molecules that have the same molecular formula but have a different arrangement of the atoms in space. That excludes any different arrangements which are simply due to the molecule's rotating ... Solved Label the molecules as cis or trans. does not apply - Chegg Label the molecules as cis or trans. does not apply trans cis trans cis does not apply does not apply trans cis trans cis does not apply Question: Label the molecules as cis or trans. does not apply trans cis trans cis does not apply does not apply trans cis trans cis does not apply This problem has been solved! See the answer
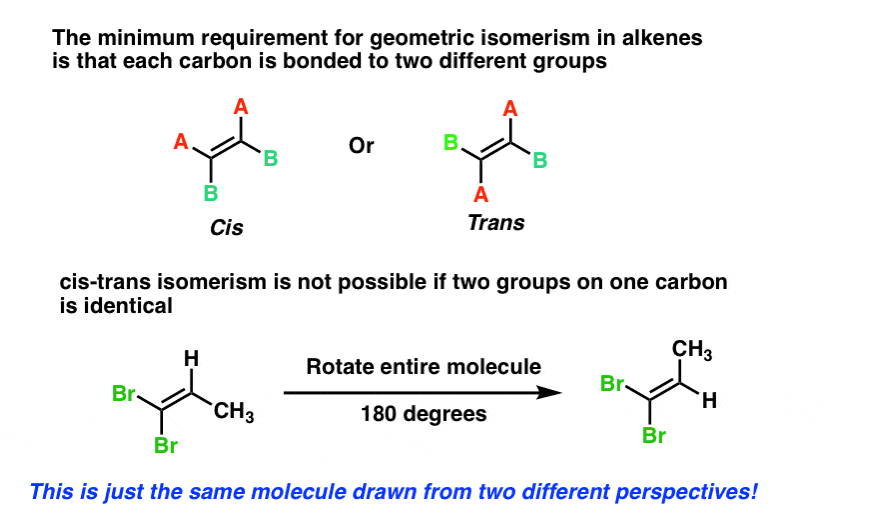
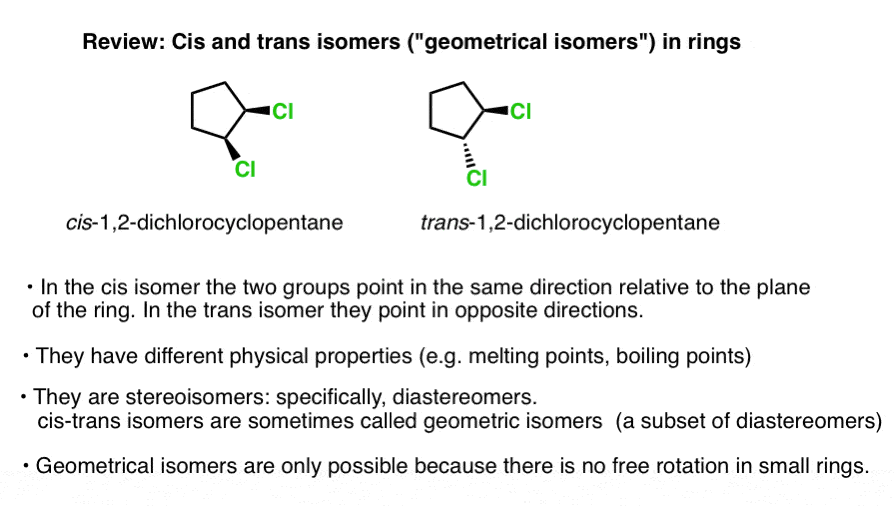
› tags › tag_labelHTML label tag - W3Schools Proper use of labels with the elements above will benefit: Screen reader users (will read out loud the label, when the user is focused on the element) Users who have difficulty clicking on very small regions (such as checkboxes) - because when a user clicks the text within the element, it toggles the input (this increases the hit area). Label the following molecules as in the cis or trans configuration ... Are the following 2 molecules structural isomers, cis-trans isomers, or not isomers?... Label each attached compound as cis or trans. Then draw the second chair conformation.... Label each compound as cis or trans. Then draw the second chair conformation.... Label each attached compound as cis or trans. Then draw the second chair conformation ... 2.3: Cis-Trans Isomers (Geometric Isomers) - Chemistry LibreTexts Cis-trans (geometric) isomerism exists when there is restricted rotation in a molecule and there are two nonidentical groups on each doubly bonded carbon atom. Exercises Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none. 2-bromo-2-pentene 3-hexene 4-methyl-2-pentene Solved Label the molecules as cis or trans. | Chegg.com Label the molecules as cis or trans. Question: Label the molecules as cis or trans. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Show transcribed image text Expert Answer 100% (84 ratings) Transcribed image text: Label the molecules as cis or trans.
Solved - Label the molecules as cis or trans. /Answer Label the molecules as cis or trans. Transcribed Image Text: Br Br A) Br B) D) Br CI does not apply does not apply does not apply does not apply cis trans cis trans trans cis trans cis. 2.99. See Answer Add To cart Related Questions. Lewis Structure for: PO4 -3 GaI3 Cl3CCF3. Geometric Isomerism Cis- and Trans- Mean in Chemistry - ThoughtCo When two substituent atoms or groups bend in the same direction, the molecule is prefixed by cis-. This molecule is cis-1,2-dichlorocyclohexane. Trans-Alicyclic Compounds Todd Helmenstine This molecule has the substituent chlorine atoms bending in opposite directions or across the plane of the carbon-carbon bond. Difference Between Cis and Trans Isomers - Isomerism - BYJUS Due to loosely packed molecules, cis isomers have relatively lower melting points than trans isomers. Due to tightly packed molecules, the melting points of trans isomers are usually higher than those of cis isomers. The boiling point of cis isomers is high due to the presence of strong forces of attraction between the atoms of the cis isomer. Which of the following compounds can exist as cis-trans isomers? b. For ... Identify the ones which show cis/trans isomerism and identify the isomer as cis or trans. Identify the given pair of compounds as stereoisomers, conformations, or constitutional isomers. Give the IUPAC names, including the cis or trans label, for each of the geometric isomers of CH_3CH_2CH=CHCH_2CH_3.
learn.microsoft.com › en-us › dotnetLabel Class (System.Windows.Controls) | Microsoft Learn A Label is a ContentControl, which means that it can contain a single object of any type (such as a string, an image, or a panel). For more information, see the ContentControl class. Customizing the Label Control. To apply the same property settings to multiple Label controls, use the Style property.
13.2: Cis-Trans Isomers (Geometric Isomers) - Chemistry LibreTexts Cis-trans (geometric) isomerism exists when there is restricted rotation in a molecule and there are two nonidentical groups on each doubly bonded carbon atom. Exercises Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none. 2-bromo-2-pentene 3-hexene 4-methyl-2-pentene
Cis and Trans - Organic Chemistry | Socratic The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon, as in but-2-ene. Then the two identical methyl groups are either cis or trans to each other, and the two identical hydrogen atoms are either cis or trans to each other.
en.wikipedia.org › wiki › LabelLabel - Wikipedia A label is a piece of paper, plastic film, cloth, metal, or other material affixed to a container or product, on which is written or printed information or symbols about the product or item. Information printed directly on a container or article can also be considered labelling. Labels have many uses, including promotion and providing information on a product's origin, the manufacturer, use, safety, shelf-life and disposal, some or all of which may be governed by legislation such as that for foo
label.foundationTRACKS – WEB 3 MUSIC STREAMING PLATFORM TRACKS shows a true decentralization and advanced ecosystem based on blockchain technology. Together with LABEL Foundation, TRACKS connects to listen and earn, create and earn. Isn’t it cool? Joining with TRACKS ecosystem with only listening to music. TRACKS shares and connects you various and amazing music through cutting edge way.
What are Cis and Trans Double Bonds? That's Simple! - Quirky Science If a molecule has more than one double bond, the molecule can have both cis and trans bonds. For example, 3,4-dimethyl-hexa-2,4-diene, with two, has one cis-double bond and one trans-double bond (see topmost image). The difference between cis and trans is not merely of intellectual value. Life chemistry requires very specific chemical structures.
Label the molecules as cis or trans. CI O does not apply O Label the molecules as cis or trans. CI O does not apply O trans O cis CI Br CI D) CI 0 0 Odoes cis trans not apply O 0 trans does not apply cis 0 0 Odoes cis trans not apply Option 1 Low Cost Option Download this past answer in few clicks 3.85 USD PURCHASE SOLUTION Option 2 Custom new solution created by our subject matter experts
Cis Trans and E Z Geometric Isomers - Leah4sci 1) First, name the alkene using the tutorial linked below. 2) Then, simply add 'cis' or 'trans' in front of the name. Take the 2 geometric isomers of 2-butene: Their proper names are as follows: When there is only one pi bond, you don't have to specify which carbon is cis or trans since. It's self-understood.
- Women's Online Clothing Boutique Shop - LABEL LABEL - Women's Online Clothing Boutique Shop - LABEL Login we're open 10am-7pm cst! Locations new arrivals every day keeping it fresh for you woman-owned small business thanks for shopping small! New Arrivals Tops Bottoms Dresses Shoes Jewelry + Accessories Gifts Under $100 Sale Gift Cards 0 Show Me Your Mumu Stanley Sweatshirt - Paradise $98.00
Solved Consider the molecule. Label molecule A as cis or - Chegg Label molecule C as cis or trans. Br trans Show transcribed image text Expert Answer 100% (25 ratings) Transcribed image text: Consider the molecule. Label molecule A as cis or trans. Br Ocis does not apply trans CI Molecule A Consider the molecule. Label molecule B as cis or trans. Ocis does not apply trans CI Br Molecule B Consider the molecule.
Cis and Trans Isomers - Chemistry Steps In this example as well, the two molecules are Cis- and trans-isomers and the absolute configuration of the chiral centers wouldn't make any difference even if it was inverted in both molecules. Reply. Dr. Twinkle. May 9, 2020 at 4:58 am . they are distereomers because one is trans and other is cis.
How can you identify cis and trans isomers? + Example - Socratic.org The difference between the two is that the 1) Cis I somer is polar whereas the T rans I somer is non − polar. 2)Cis isomer due to being polar shows Dipole moment while the trans isomer does not. Example: Consider the case of 1,2-dichloroethene In one, the two chlorine atoms are locked on opposite sides of the double bond.
(Get Answer) - Label the molecules as cis or trans. does not apply ... Geometric isomers are not restricted to compounds containing the C C bond. For example, certain disubstituted cycloalkanes can exist in the cis and the trans forms. Label the following molecules as the cis and trans isomer, of the same...
Answered: Label the molecules as cis or trans. Br… | bartleby Solution for Label the molecules as cis or trans. Br Br A) B) D) Br Br CI does not apply does not аpply does not аply trans cis trans cis trans does not apply…
Label the molecules as cis or trans. Trans cis does not apply trans ... Answer 2:- C) Cis-2-pentene, because its molecules cannot stack as efficiently Explanation:- in melting point... Answer true or false. (a) Both ethylene and acetylene are planar molecules. (b) An alkene in which each carbon of the double bond has two different groups bonded to it will show cis-trans isomerism.
Solved Label the molecules as cis or trans. Trans cis - Chegg Question: Label the molecules as cis or trans. Trans cis does not apply trans does not apply cis trans cis does not apply trans does not apply cis This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Show transcribed image text Expert Answer 100% (5 ratings)
Answered: Label the molecules as cis or trans. Br… | bartleby Label the molecules as cis or trans. Br B) CI 2 Br 6 Br D) -0 Br Question Transcribed Image Text: Label the molecules as cis or trans. Br B) CI does not apply trans cis Br trans cis does not apply 6 Br cis does not apply trans D) -G Br does not apply trans cis Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution
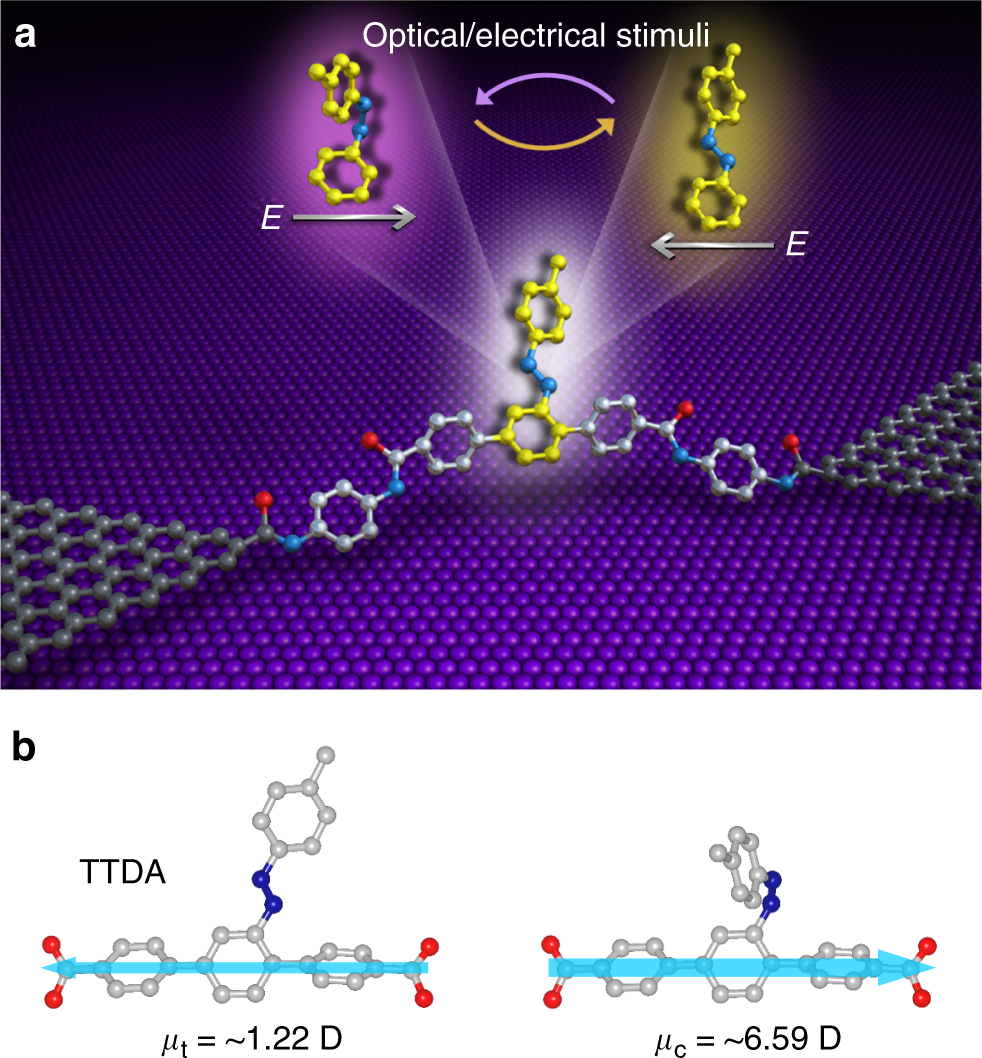


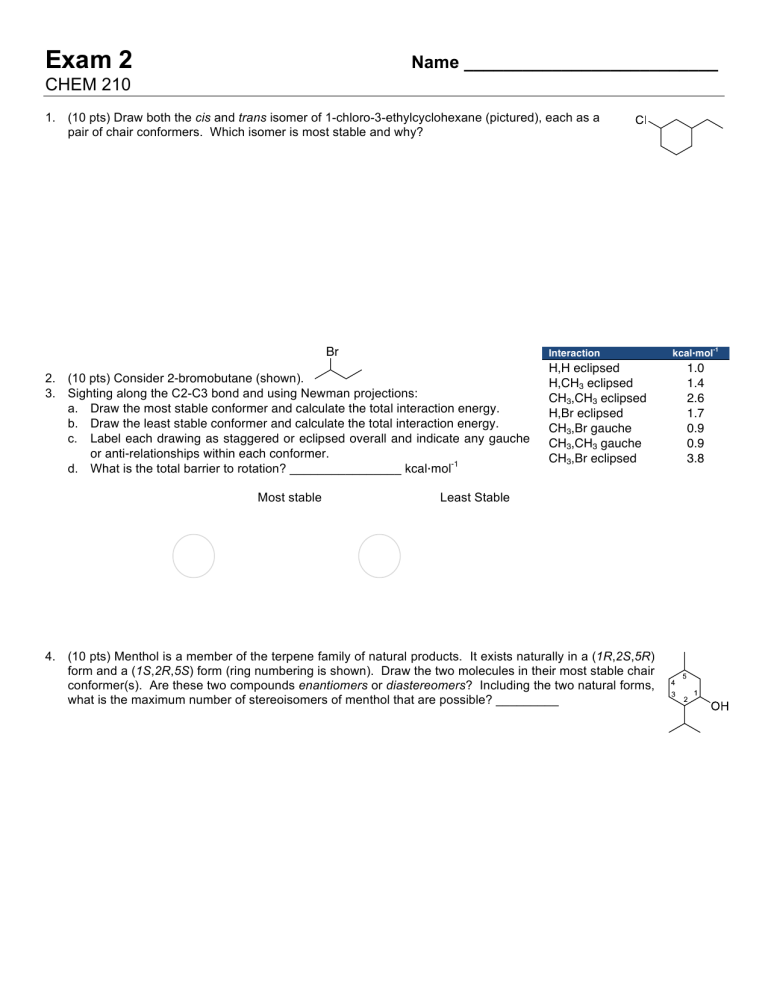
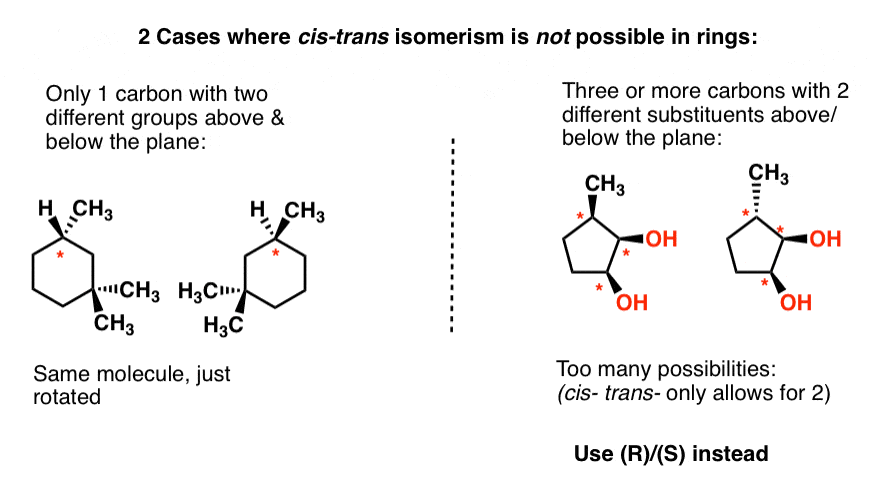

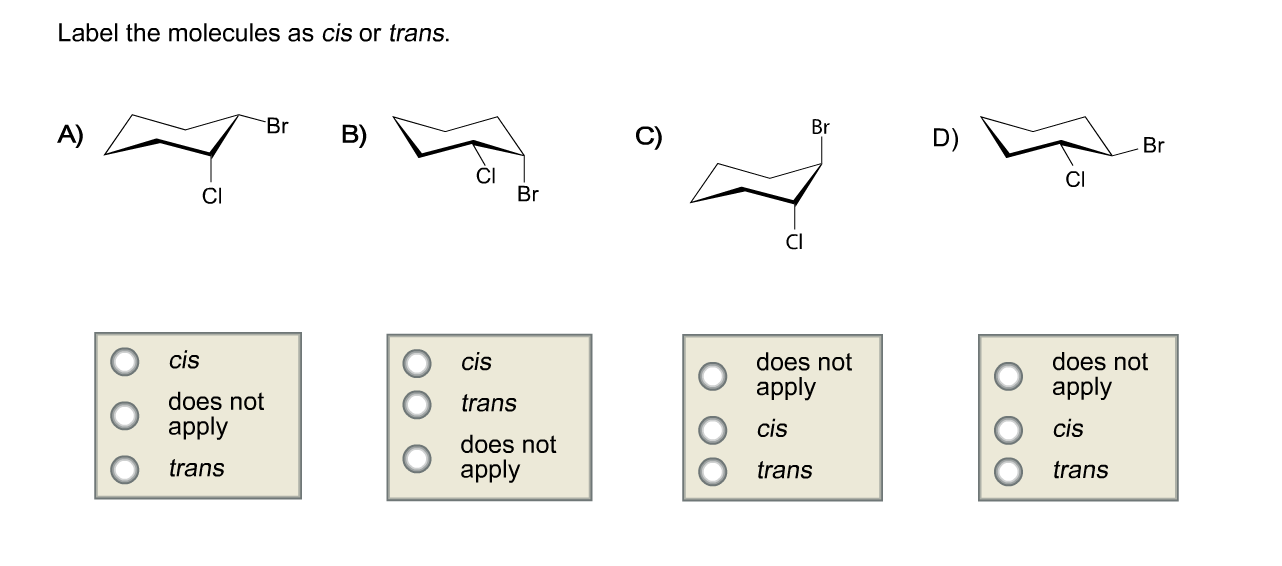

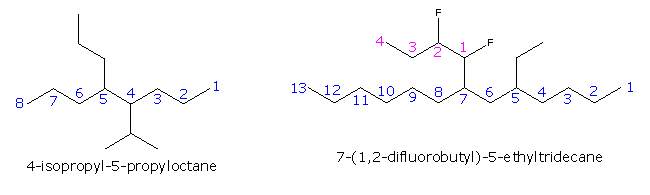
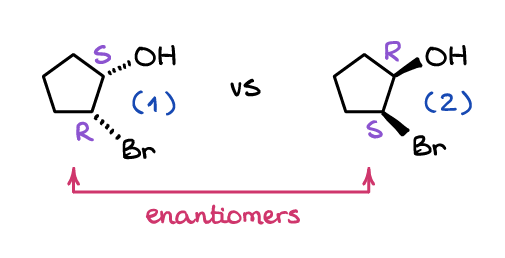
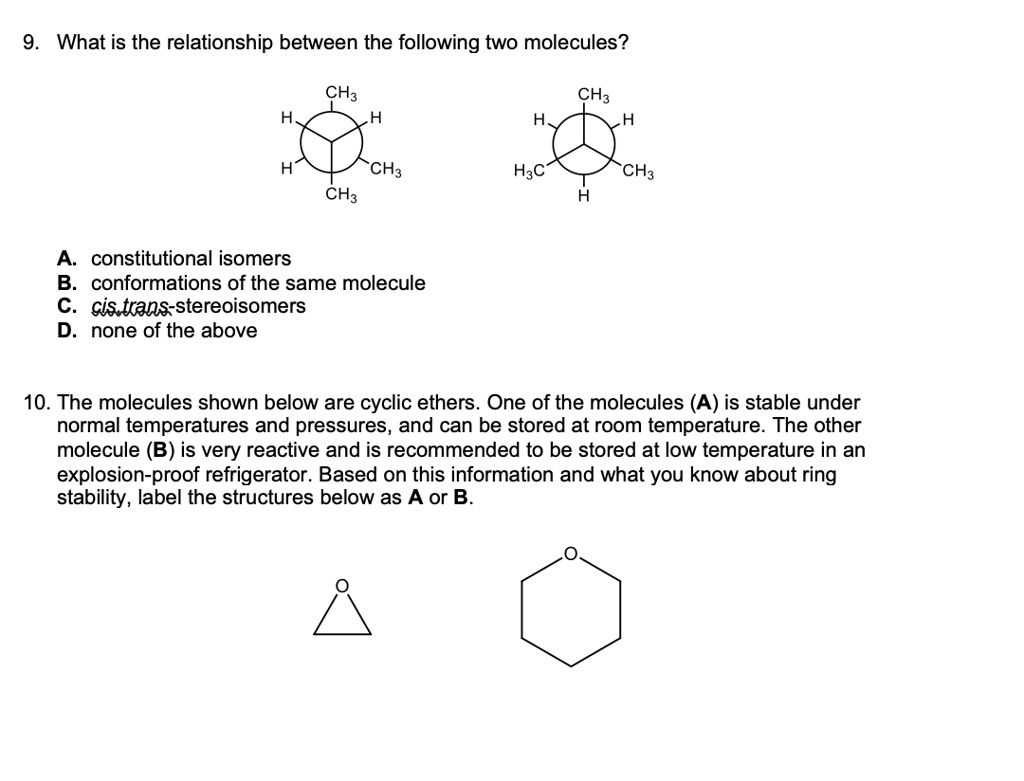


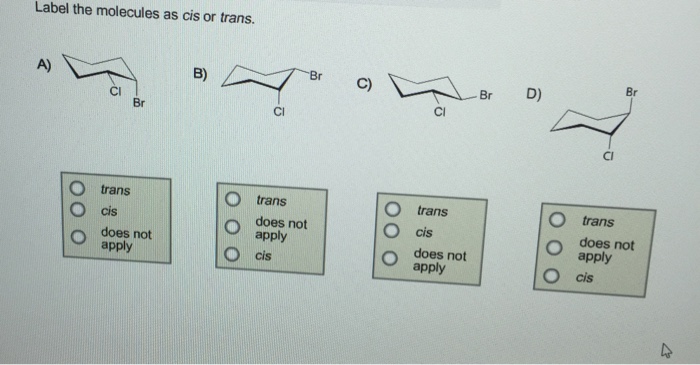


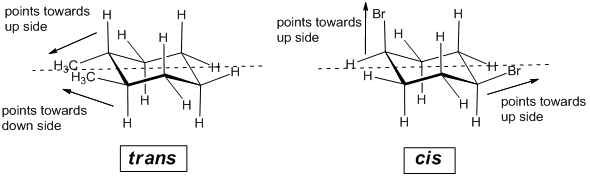


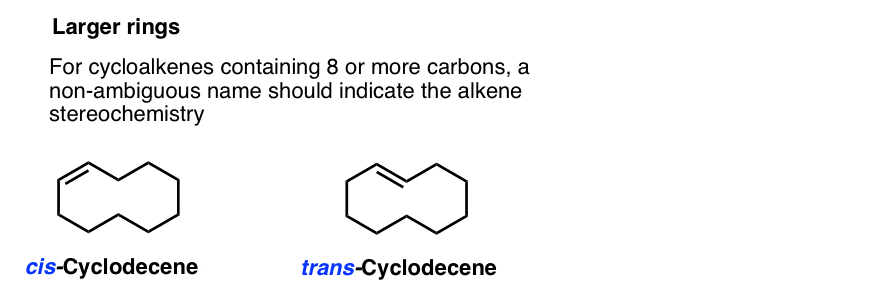










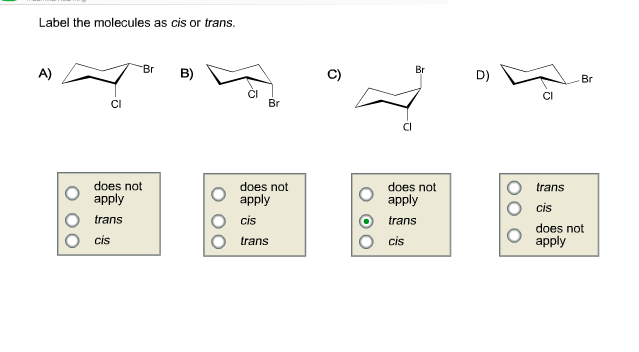
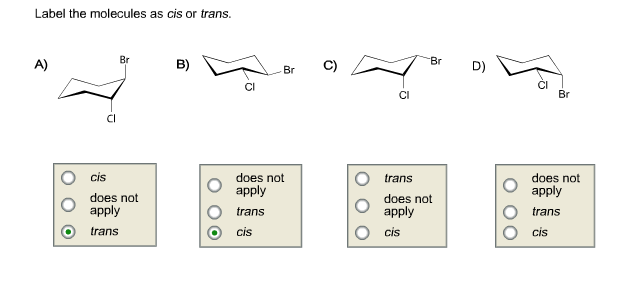
Post a Comment for "43 label the molecules as cis or trans."