43 multistep reaction energy profile
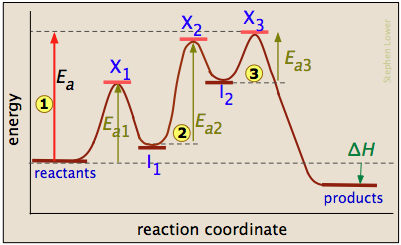
AP Chemistry Unit 5 Notes - The rates of ___________ of reactant and ... The energy profile gives the energy along the reaction coordinate, which typically proceeds from reactants, through a transition state, to products. ... Multi-Step Reaction Energy Profiles Knowledge of the energetics of each elementary reaction in a mechanism allows for the construction of an energy profile for a multistep reaction. AP Chemistry - Unit 5: Kinetics I can identify the rate law for a reaction in which the first step is not rate limiting. Topic 5.10: MultiStep Reaction Profile. I can represent the activation energy and the overall energy in a multistep reaction with a reaction energy profile. Topic 5.11: Catalysis. I can explain how catalysts affect the reaction rate by changing the reaction ...
Multi-Step Reaction - an overview | ScienceDirect Topics Step-wise reactions generally require heating whereas chain processes are generally run below room temperature and normally require cooling. Chain polymerizations are generally exothermic and more rapid than the step-wise reactions. The difference in the reaction speed is a result of the differences in activation energy for the controlling step.

Multistep reaction energy profile
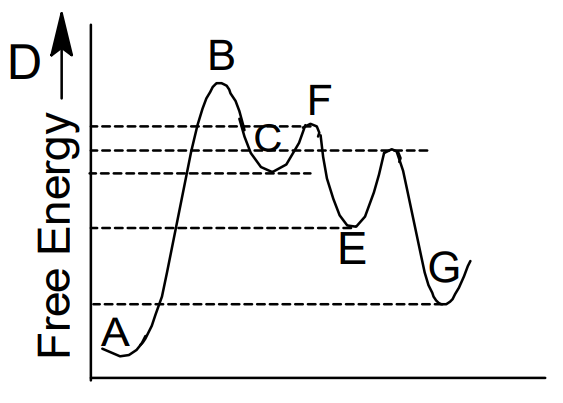
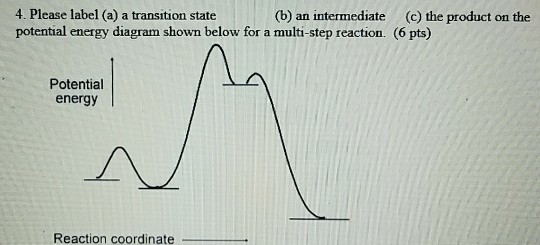
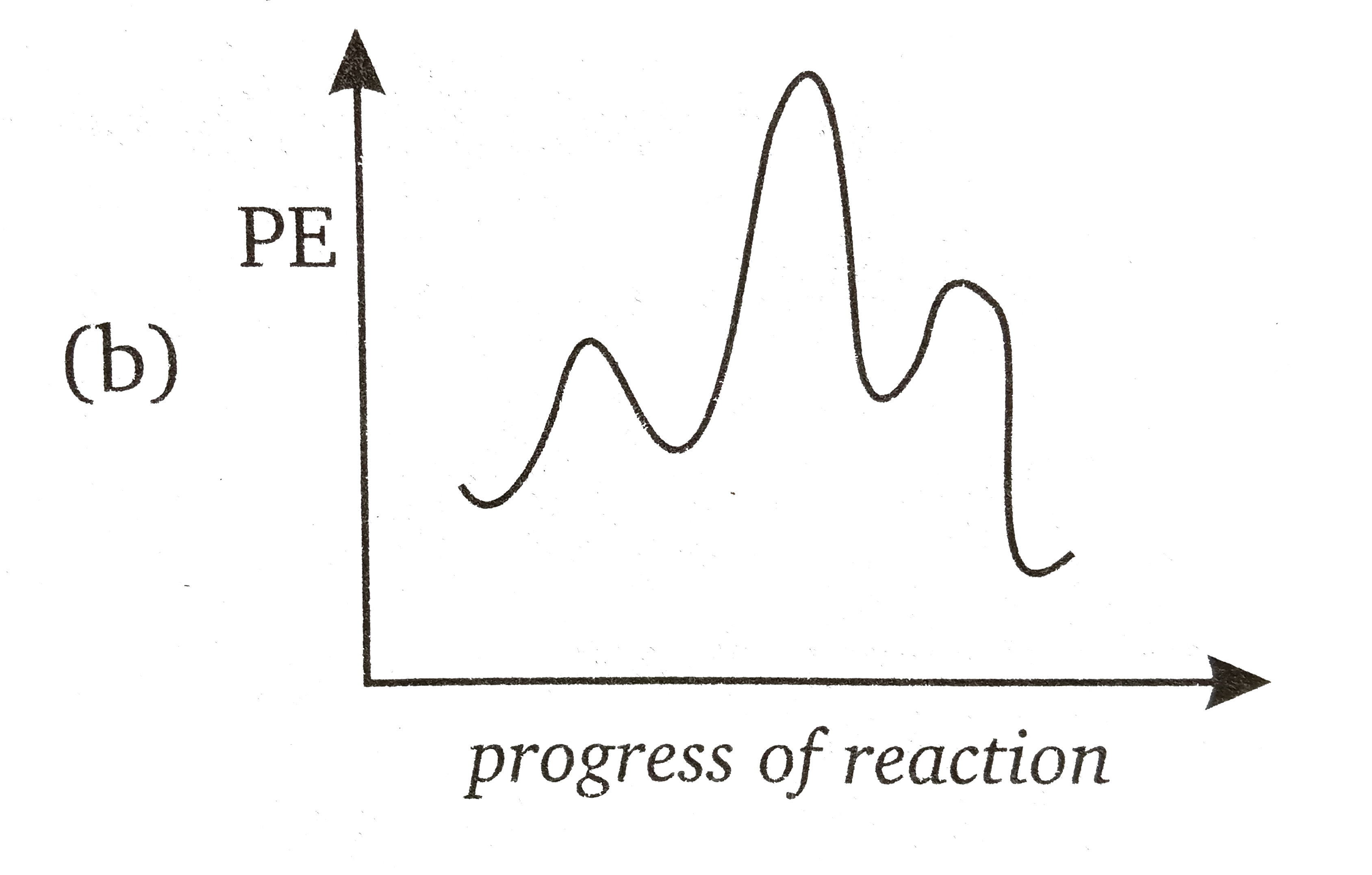
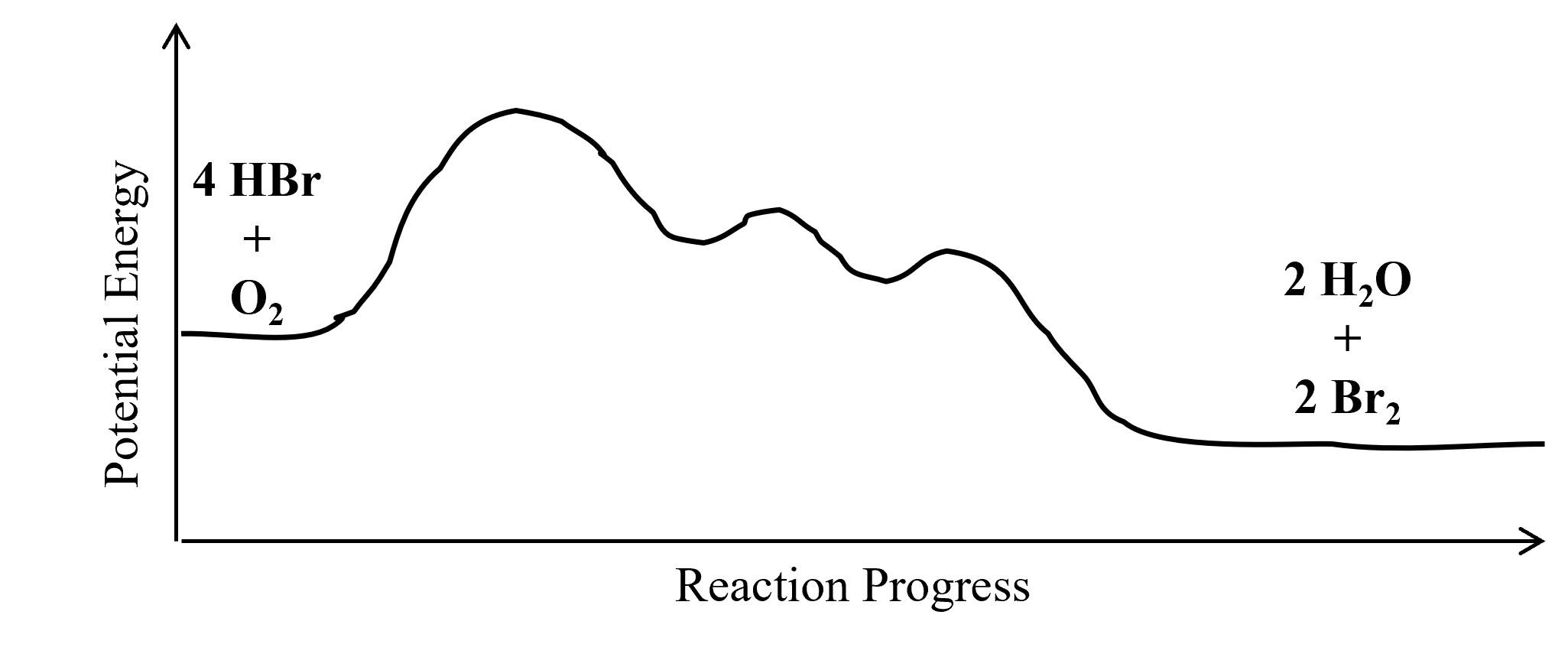
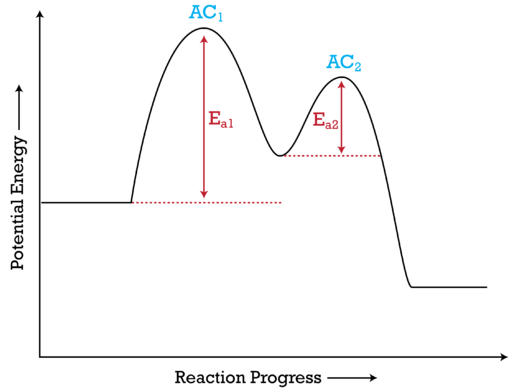
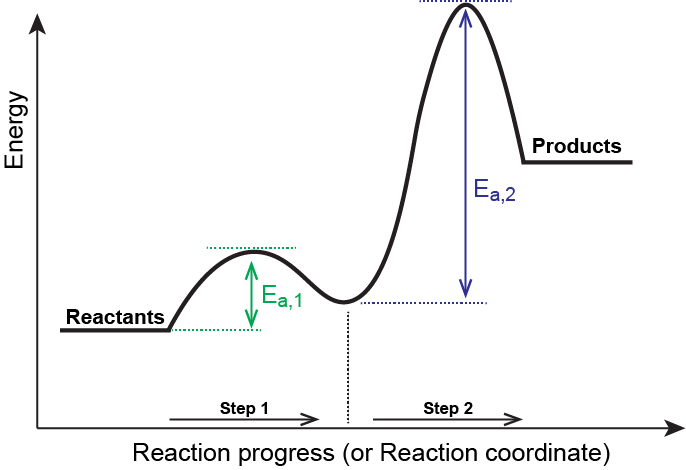
Multistep Reaction Energy Profile | StudyAPChemistry In addition, the energy of the reactants won't always be higher than that of the products. This is an exothermic reaction, as heat energy is released. We will cover this more in the next unit, which is Thermodynamics. That's basically it for the Multistep Reaction Energy Profile. 5.10+Multistep+Reaction+Profile+Diagram+key.student.pdf NOTES: Reaction energy profiles can also be used to illustrate a multistep reaction, as long as the energetics for each step are known. In a two-step reaction, two transition states are shown. In a three-step mechanism, three transition states are shown. 5.10 - Multistep Reaction Energy Profiles - YouTube 5.10 - Multistep Reaction Energy Profiles Shanna Barkume 1.09K subscribers Subscribe 8 Share 1.2K views 2 years ago Notes graphic organizer can be found at:...
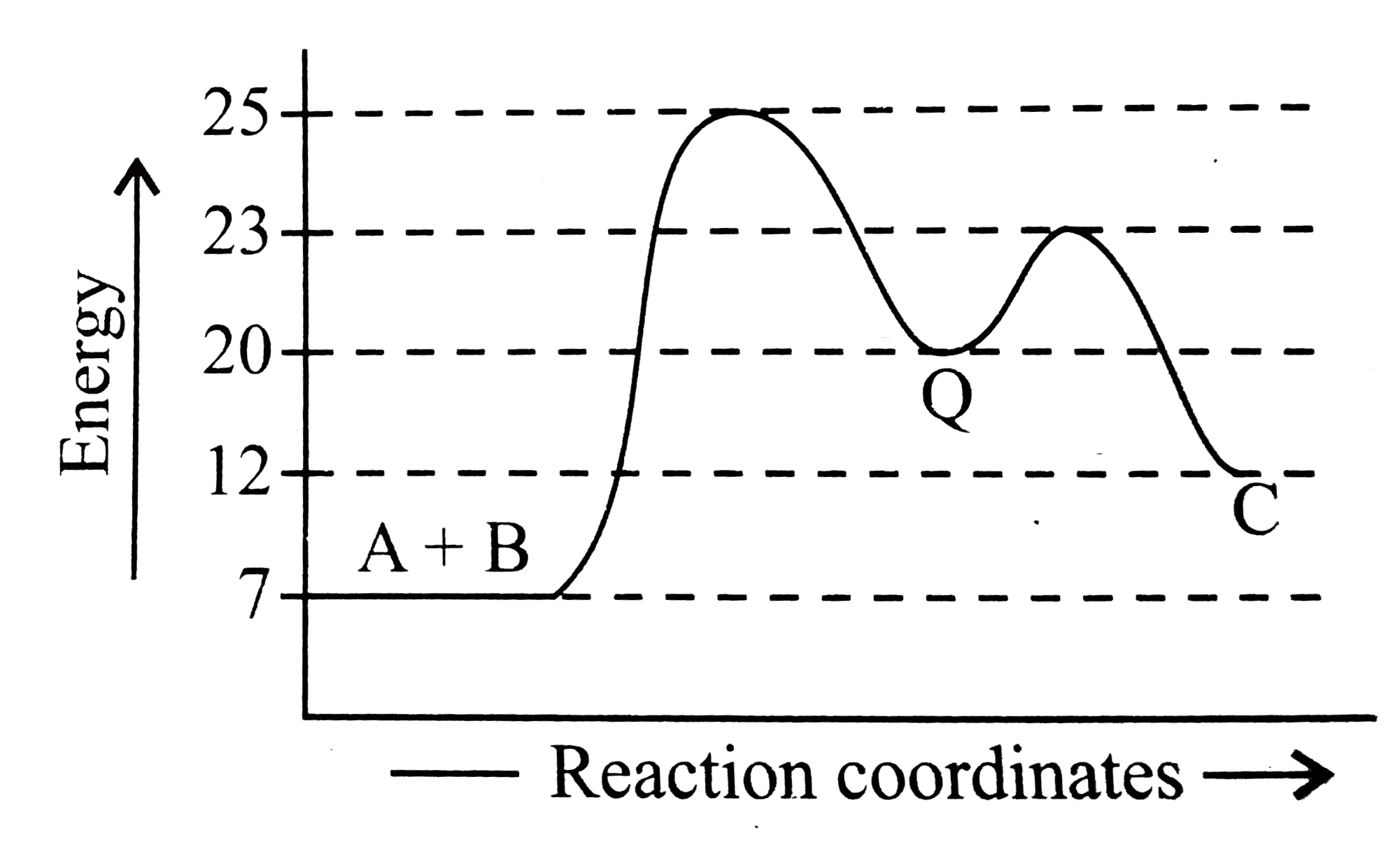
Multistep reaction energy profile. AP Chemistry: 5.10 Multisteps Reaction Energy profile - Exam Style ... (A) The activation energy for step 1 of the mechanism is large and positive. (B) The activation energy for step 2 of the mechanism is small and positive. (C) The value of\( \Delta S^{\circ}\) for the overall reaction is small and positive. (D) The value of \(\Delta H^{\circ}\) for the overall reaction is large and negative. Answer/Explanation ... Energy Profile: Definition, Diagram, Reaction | StudySmarter Energy Profile Chemical Analysis Formulations Instrumental Analysis Pure Substances Sodium Hydroxide Test Test for Anions Test for Metal Ions Testing for Gases Testing for Ions Chemical Reactions Acid-Base Reactions Acid-Base Titration Bond Energy Calculations Decomposition Reaction Electrolysis of Aqueous Solutions Electrolysis of Ionic Compounds Multistep reaction energy profiles (video) | Khan Academy having trouble loading external resources our website. you behind web filter, please make sure that the domains .kastatic.org and .kasandbox.org are unblocked. CoursesMath Pre 8th gradePre through grade Khan Kids Early math... Multistep Reaction: Definition & Energy Profile | StudySmarter Multistep Reaction Chemical Analysis Formulations Instrumental Analysis Pure Substances Sodium Hydroxide Test Test for Anions Test for Metal Ions Testing for Gases Testing for Ions Chemical Reactions Acid-Base Reactions Acid-Base Titration Bond Energy Calculations Decomposition Reaction Electrolysis of Aqueous Solutions
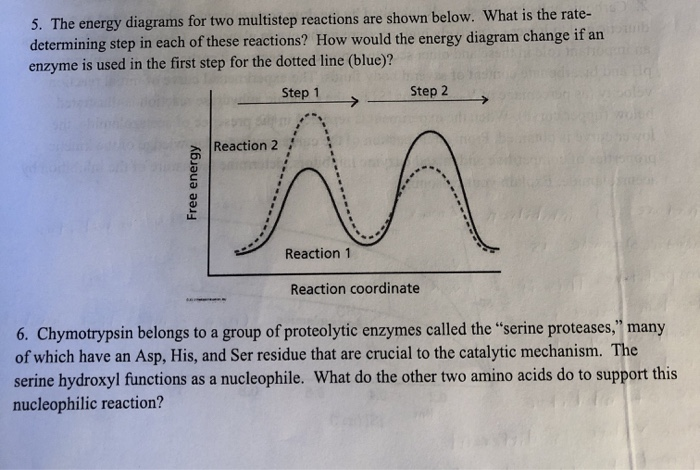
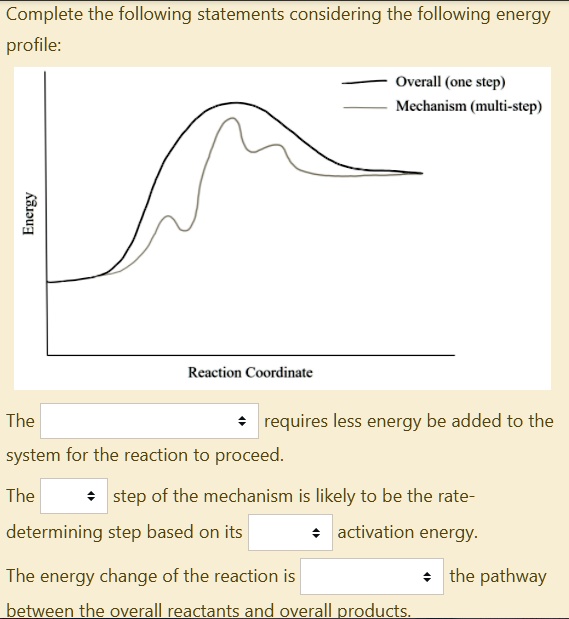
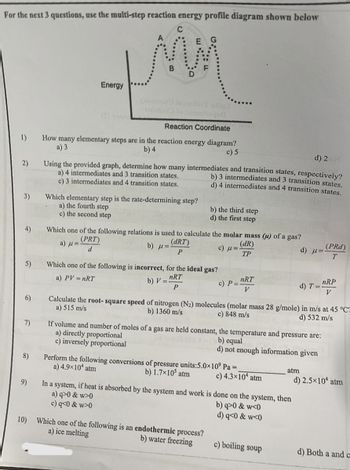
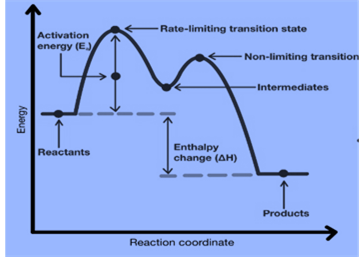
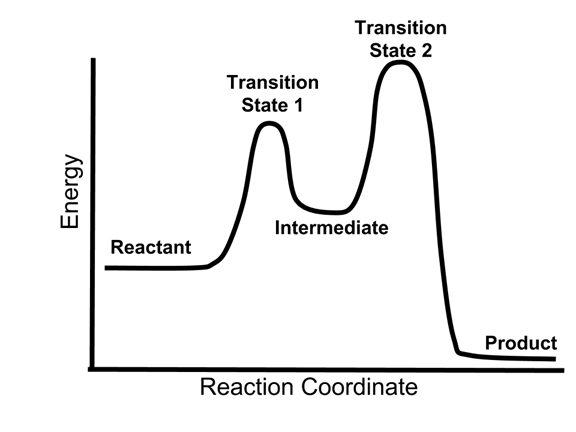
Multistep reaction energy profiles (video) | Khan Academy The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction. Created by Jay. Sort by: Top Voted Questions Tips & Thanks Emma a year ago at 0:08 5.10 Multistep reaction energy profiles.pdf - Course Hero 5.10 Multistep reaction energy profile Review: Most reactions have multiple stepsThe rate of reaction is determined by the slowest step In a single step reaction, you see a simple energy diagram like the one on the right: In multi-step reactions, each elementary step has its own activated complex and its own activation energy barrier. Reaction mechanism and rate law (article) | Khan Academy A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step. The slowest step in a reaction mechanism is known as the rate-determining step. Multistep reaction energy profiles | Kinetics | AP Chemistry | Khan ... Multistep reaction energy profiles | Kinetics | AP Chemistry | Khan Academy Khan Academy 7.53M subscribers 10,666 views Jan 30, 2021 Keep going! Check out the next lesson and practice what...
What is multistep reaction? - Short-Question The energy profile for a multistep reaction can be used to compare the activation energies of the different steps and identify the rate-determining step. The energy profile can also be used to determine the overall change in energy for the reaction. What are the 2 steps of a chemical reaction? 5.10 - Multistep Reaction Energy Profiles - YouTube 5.10 - Multistep Reaction Energy Profiles Shanna Barkume 1.09K subscribers Subscribe 8 Share 1.2K views 2 years ago Notes graphic organizer can be found at:... 5.10+Multistep+Reaction+Profile+Diagram+key.student.pdf NOTES: Reaction energy profiles can also be used to illustrate a multistep reaction, as long as the energetics for each step are known. In a two-step reaction, two transition states are shown. In a three-step mechanism, three transition states are shown. Multistep Reaction Energy Profile | StudyAPChemistry In addition, the energy of the reactants won't always be higher than that of the products. This is an exothermic reaction, as heat energy is released. We will cover this more in the next unit, which is Thermodynamics. That's basically it for the Multistep Reaction Energy Profile.


























Post a Comment for "43 multistep reaction energy profile"